Where Did The Europeans Get Their Domesticated Animals From
- Research article
- Open Access
- Published:
The genetic prehistory of domesticated cattle from their origin to the spread beyond Europe
BMC Genetics volume 16, Article number:54 (2015) Cite this article
Abstract
Background
Cattle domestication started in the 9th millennium BC in Southwest Asia. Domesticated cattle were and then introduced into Europe during the Neolithic transition. Yet, the scarcity of palaeogenetic data from the outset European domesticated cattle nevertheless inhibits the accurate reconstruction of their early on demography. In this study, mitochondrial Deoxyribonucleic acid from 193 ancient and 597 modernistic domesticated cattle (Bos taurus) from sites beyond Europe, Western Anatolia and Iran were analysed to provide insight into the Neolithic dispersal procedure and the function of the local European aurochs population during cattle domestication.
Results
Using descriptive summary statistics and series coalescent simulations paired with approximate Bayesian computation we discover: (i) decreasing genetic diversity in a southeast to northwest direction, (two) strong correlation of genetic and geographical distances, iii) an estimated constructive size of the Near Eastern female founder population of 81, iv) that the expansion of cattle from the About East and Anatolia into Europe does not appear to constitute a meaning bottleneck, and that v) there is evidence for gene-flow between the Near Eastern/Anatolian and European cattle populations in the early phases of the European Neolithic, just that it is restricted after 5,000 BCE.
Conclusions
The about plausible scenario to explain these results is a unmarried and regionally restricted domestication process of cattle in the Near Due east with subsequent migration into Europe during the Neolithic transition without significant maternal interbreeding with the endogenous wild stock. Show for gene-menstruum between cattle populations from Southwestern Asia and Europe during the earlier phases of the European Neolithic points towards intercontinental trade connections between Neolithic farmers.
Background
The transition from foraging to producing economies, as well chosen Neolithisation, was a major turning-point in human prehistory. The process of Neolithisation started in a region spanning from the Zagros Mountains to Fundamental Anatolia and from Palestine to the plains beyond the E Taurus Mountains [1,2]. It was characterized by the successive appearance of sedentism (12th-tenthursday millennia BCE), constitute cultivation (mid-10th millennium), beast husbandry (mid-nineth millennium) and pottery (early seventh millennium) [iii,4]. Elements of the Neolithic lifestyle expanded into Western Anatolia in the early 7th millennium [5-7], while the earliest signs for Neolithic settlements on the European continent are found in present-solar day Hellenic republic around half-dozen,400 BCE [viii]. The subsequent Neolithic spread across the balance of Europe followed at least two master routes: I leading across Southeastern Europe, and the 2nd via the Western Mediterranean [nine-11]. The extent to which this expansion of a new civilisation and economic system was driven past the migration of people has been debated for decades [12-17]. An early study on homo ancient Dna emphasized the office of inwards migration at the showtime of the Neolithic period in Central Europe [18], a view that is supported past more than contempo palaeogenomic studies [19,20].
As animate being husbandry was an important function of the foundation of the new agricultural lifestyle, remains of domesticated animals can serve as a good proxy for the Neolithic spatial expansion and the presence and activity of farmers in newly populated areas [21]. In contempo years, genetic and palaeogenetic studies have increasingly converged on a Southwest Asian origin for the four Neolithic domesticated animals: cattle, sheep, goats, and pigs [22]. For Near Eastern taurine cattle (Bos taurus), a recent coalescent-based analysis using ancient Iranian samples suggested a severe Virtually Eastern domestication bottleneck, with an estimated effective size of merely lxxx female founders [23]. Even so, comprehensive data sets of aboriginal cattle Dna from other areas are and so far restricted to Central and Western Europe, for example from Bollongino et al. [24]. Thus, detailed and continent-broad evaluation of the early spatiotemporal census of Bos taurus has and so far been hindered by the lack of information from the fundamental bridging areas of the Neolithic, namely Anatolia, the Balkans, and the Western Mediterranean.
In this study we greatly extend a previous coalescent-based demographic model, based on 15 ancient Iranian and 27 modernistic Near Eastern and Anatolian cattle mitochondrial DNA (mtDNA) sequences, in terms of sample size, and geographic and temporal range [23]. To be able to investigate the early population history and migratory patterns of taurine cattle in detail, the present model is now conditioned on a larger aboriginal (n = 193, including the Iranian samples) and modern (n = 597) mtDNA dataset that widely covers the surface area of the Neolithic westwards expansion from the 7th millennium BCE onwards. The focus of the study is on the time period when cattle were first introduced to Europe, thereby allowing usa to accost the following questions: i) Is the scenario of a single and astringent domestication bottleneck in the Near East still supported when adding the much larger dataset from western Anatolia and Europe? ii) Did cattle attain Europe in a single dispersal procedure or is at that place prove for multiple introductions or continuous factor-flow between regions? 3) How much of the genetic diversity from the Near East was introduced to the European continent? four) Did the spread of cattle coincide with the spread of the Neolithic culture? and v) Are there any signs of admixture with female aurochs along the expansion road of domestic cattle?
Methods
Textile
150 samples of prehistoric domestic cattle from 24 archaeological sites were taken to analyse a 434 bp long mitochondrial d-loop fragment (for detailed information on the archaeological sites and sample age see Boosted file one. The majority of investigated individuals (113) come from Western Anatolia and Southeastern Europe, i.e. a region defined as an "acting zone" [v], pointing to its bridging position between the "Neolithic core zone" and its European fringe.
A further 22 samples from Southern French republic and Southern Italy stand for the kickoff domesticated cattle to reach Europe on the "Mediterranean route" of the Neolithic expansion.
Additionally, new prehistoric samples from Germany (4), Northern and Western French republic (11) and Syria (1), plus 80 previously published sequences mainly from Central and Western Europe and Iran were used for population genetic analyses ([23-28] and GenBank: KC172647 - KC172649). A total of 597 modern d-loop sequences of 240 bp length were collected from previously published studies [29,thirty]. They each provide representative sets of sequences that lucifer the European, Anatolian and Near Eastern written report area of the present paper, thereby also covering areas which are underrepresented in the aDNA dataset, e.g. Italy and the Iberian Peninsula. For a complete list of GenBank accession numbers of previously published sequences run across Additional file ii.
Ancient DNA work and sequencing
All samples were processed in the ancient Deoxyribonucleic acid facilities at the Establish of Anthropology, Mainz Academy (Germany), nether strict rules for contamination prevention as described in Bramanti et al. [18]. Those include strict separation of pre-PCR and mail service-PCR labs, protective dress, regular cleaning of surfaces and equipment with detergent and bleach, and UV-irradiation of rooms, laboratory hoods, and equipment. Bone samples were UV-irradiated for 45 min from two sides. The surface was mechanically removed using a sandblaster (P-G 400, Harnisch & Rieth) or rotary saw (Electer Emax IH-300, MAFRA). Os cubes of about 0.3 cm side length were over again UV-irradiated for 45 min from 2 sides. Samples were pulverized using a mixer mill (MM200, Retsch). Generally, aliquots of 0.5 grand bone powder were incubated on a rocking shaker at 37°C in a decalcification and digestion solution containing two.5 ml EDTA (0.5 M, pH8; Ambion®/Applied Biosystems), 250 μl Northward-Laurylsarcosine (0.5 %; Merck) and 30 μl Proteinase K (18 U; Roche). DNA extraction was performed using phenol-chloroform-isoamylalcohol (25:24:1; Roth). Deoxyribonucleic acid was washed and full-bodied using l kDa Centricons or 50 kDa fifteen ml Amicons (Millipore). At least two independent extractions per sample were performed. Extraction blank controls were processed during each extraction. Additionally, the cleanness of the grinding jars was tested past extracting hydroxylapatite that was pulverized nether the same weather as the bone samples.
Distension of 434 bp of the HVSI (positions 15914–ix according to reference sequence V00654) was more often than not conducted using a PCR primer fix consisting of half-dozen primer pairs every bit in Bollongino et al. [23] (BosU1/L1-BosU6/L6) with slight modifications.
PCR reactions were usually performed with two.5 U AmpliTaq Gold® (Applied Biosystems), 1x PCR Gilded Buffer (Applied Biosystems), 2 mM MgCl2 (Applied Biosystems), 0.2 mM dNTP's (Quiagen), 0.4 μg/μl BSA (Roche), and 0.2 μM primer (Biospring) and HPLC-H20 (Acros Organics). Initial activation at xc°C for vi min was followed by 50 cycles of denaturation (xl sec at 94°C), annealing (40 sec at 52-sixty°C), and elongation (40 sec at 72°C) in a Mastercycler gradient (Eppendorf). Blank controls were candy during each PCR. At to the lowest degree three contained PCRs from two different extracts were performed. Samples were sequenced on an ABI PRISM™ 3130 Genetic Analyzer (Applied Biosystems) using Pop-half-dozen™ polymer (Applied Biosystems).
Sequences were analysed using the programs SeqMan™ and MegAlign™ (DNASTAR Lasergene® seven.1 and 8). At least iii sequences obtained from independent PCRs from ii independent DNA extractions per sample per primer pair were usually used to create a majority rule consensus sequence. For further details on aboriginal Deoxyribonucleic acid work and sequencing including deviations from the full general laboratory procedure described see Additional file 3.
Descriptive and summary statistics
All new and previously published ancient Dna sequences were subdivided into the following geographical groups: Iran/Syria, Western Anatolia, Southeastern Europe, Southeastern Central Europe, Italian republic, Southern France, Central/Western Europe, and Spain. These groups were further subdivided into chronological subgroups reflecting up to iv different Neolithic and post-Neolithic periods per region (come across Boosted file 4 for detailed information on the groupings). Modern sequences were grouped co-ordinate to their state of origin (too see Additional file 2).
For statistical analyses, all ancient sequences were cutting to a 399 bp fragment to match the fragment sizes of previously published aboriginal sequences (positions 15,914-sixteen,312 according to reference sequence GenBank V00654). Haplotype variety, mean number of pairwise differences, Tajima's D, Fu'southward Fs and population pairwise FST were calculated using Arlequin 3.5.1.ii [31]. P values are based on ten,000 random permutations. The level of missing information immune was adjusted in order to include all nucleotide positions even if there were gaps in some ancient sequences. Besides that, default values were used.
The MDS (multidimensional scaling) plot is based on FST values calculated using Reynolds' genetic distances and running 10,000 permutations in Arlequin 3.five.ane.2. The MDS plot was created in R ii.14.2 R [32] using the packages MASS [33], plotrix [34] and shape [35].
Correlation between genetic and geographical distances among defined populations/groups was assessed by a Mantel test [36] under 9,999 random permutations using GENALEX 6.4 [37]. The Mantel examination is based on FST values calculated using Reynolds' genetic distances and running ten,000 permutations in Arlequin three.five.1.two. Geographical coordinates were determined by eye as the centre of advisable countries per grouping for the modern samples and the centre of all archaeological sites per group for the ancient samples.
Coalescent simulations
Coalescent simulations were performed using Bayes Serial SimCoal [38], by extending the model previously described in Bollongino et al. [23]. Similarly, nosotros assume an intergeneration fourth dimension of 6 years, an ancestral Near Eastern wild aurochs female person effective population size of 45,000 [39] and, again, a single domestication process of parameterized size N D at time 8,500 years BCE (i.due east. one,750 generations BP). Following the domestication bottleneck, this Near Eastern population grows exponentially to a modern Nearly Eastern and Anatolian constructive population size Northward NE of one,007,170 (see SI Bollongino et al. [23]). At 6,400 years BCE (i.e. i,400 generations BP) a proportion of the population P is immune to migrate to class a new and split up European population, which then grows exponentially to a modern European effective population size Due north E of 7,942,392 (boosted simulations which allow both N NE and N E to vary by an gild of magnitude are described further in Additional file v). From the carve up fourth dimension until 5,000 years BCE migration between the two populations is allowed at per generation rate M E ('early migration'), after which it is inverse to charge per unit Yard 50 ('belatedly migration'). Prior values for North D are fatigued uniformly from the range 1 – 1,000, P uniformly from the range 0 – one, and both migration parameters uniformly from the range 0 – 0.01. The mutation charge per unit is fixed at 45% per million years, the posterior modal value previously estimated past Bollongino et al. [23].
Nosotros used the in a higher place-mentioned 597 previously published modern sequences of 240 bp length (positions 16,023-16,262 co-ordinate to reference sequence GenBank V00654) and cut the ancient sequences accordingly. The resulting 790 sequences were grouped into 4 sample groups: ancient About Eastern and Anatolian (n = 24), ancient European (n = 169), modernistic Virtually Eastern and Anatolian (n = 100) and modernistic European (north = 497). Nosotros calculated 5 within- and 2 between-sample summary statistics (total = 32, as well see Additional file 5 for details), and used approximate Bayesian computation (ABC [40]) to approximate parameter values.
Results
Out of 150 newly analysed bones and teeth from prehistoric domesticated cattle, 113 yielded replicable and highly reliable mitochondrial HVR1 sequences, constituting a success rate of 75.3%. The sequences have been deposited in GenBank [GenBank: KF307209 to KF307322]). None of the blank controls independent amplifiable amounts of bovine Deoxyribonucleic acid (for further detailed discussion of the validity of the ancient DNA data encounter Additional file six). The successfully analysed samples come from Bosnia-Herzegovina (3 of 5), Bulgaria (52 of 68), French republic (xv of 19), Germany (4 of four), Italy (five of fourteen), Romania (fifteen of 16), Syria (1 of 1), and Turkey (18 of 23).
Using the nomenclature of Achilli et al. [41], all sequences belong exclusively to lineages from haplogroups that have previously been defined in present-twenty-four hours European domesticated cattle, namely T3 (n = 70), Q (n = 33), T2 (n = half-dozen) and T, T5 or T1'2'3 (north = 4). None of them belongs to a specific mtDNA motif referred to equally haplogroup P that is dominating in the ethnic aurochs population of Europe [26,27,42]. Information technology is of note that the high frequency of haplogroup Q in ancient Southeastern Europe (betwixt 50% and 29% in 5,500-5,000 BCE and 2,700-two,200 BCE, respectively) does not match nowadays-day haplogroup distributions of taurine cattle from Europe, and particularly from the same surface area (combined frequency for T and Q in present-day Balkan and Hellenic republic: 1.v - ii% [43]). It is likewise markedly higher than in all other ancient European groups (east.g. only 4% in Primal/Western Europe (five,400-four,400 BCE)). See Additional file four for item on haplogroup composition and frequency of haplogroup Q across the 13 spatiotemporal groups.
There are 35 unlike mitochondrial lineages in the 193 prehistoric individuals, eight of which occur more than one time in the dataset. Non-unique haplotypes (H) were named according to their haplogroup and numbered consecutively (H1-H8). Boosted files 4 and 7 provide a detailed overview on the distribution of haplogroups and shared and unique haplotypes across the xiii spatiotemporal groups. But haplotypes called T3_H1, Q_H4, and T2_H7 occur more than twice in the dataset (114, 37, and 5 times, respectively), with T3_H1 also being predominant in nowadays-day taurine cattle. Haplotype T3_H1 occurs in all of the ancient 13 spatiotemporal groups, Q_H4 in all except Spain ii,700-i,600 BCE and Southern French republic 5,500-4,500 BCE. It is of note that Q_H5, T_H6, and T2_H7 are restricted to the geographical groups of Iran/Syrian arab republic and Southeastern Europe (Q_H5 in Iran/Syria 4,000-1,400 BCE and Southeastern Europe half dozen,200-5,500 BCE; T_H6 in Iran 7,000-5,000 BCE and Southeastern Europe 2,700-two,200 BCE; T2_H7 in Islamic republic of iran 7,000-5,000 BCE and Southeastern Europe v,500-5,000 BCE).
Genetic distances between cattle populations
The MDS plot (Figure 1) reveals a pattern that separates three geographical groups: The four Southeastern European groups cluster with the one from Western Anatolia; both groups from Islamic republic of iran/Syrian arab republic and from Central/Western Europe are close to each other. However, Southern French republic and Central/Western Europe are isolated from all other groups and from each other.
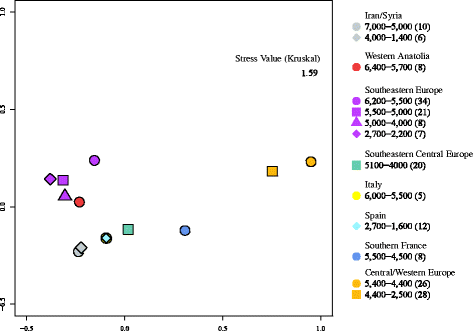
MDS Plot of d-loop sequences from thirteen spatiotemporal groups of ancient domesticated cattle. The MDS plot is based on Reynolds' FST. Numbers represent the age of samples in BCE per group; brackets incorporate the number of sequences per group.
Subgroups comprising only the earliest Neolithic cattle of each geographical group were used to further evaluate the influence of sample age and geographical location on genetic distances. Effigy 2 maps significant pairwise FST values betwixt the resulting eight groups. The greatest genetic distances can be observed between Iran 7,000-5,000 BCE and the groups from Cardinal/Western Europe 5,400-4,400 BCE and Southern France 5,500-4,500 BCE with values of 0.47 and 0.40, respectively. These groups also show the greatest geographical distances. The 2nd highest FST values occur betwixt Southeastern Europe 6,200-5,500 BCE and Primal/Western Europe 5,400-4,400 BCE and between Southeastern Europe half-dozen,200-5,500 BCE and Southern France 5,500-iv,500 BCE (0.27 and 0.29 respectively). In comparing, the genetic distance between Islamic republic of iran 7,000-5,000 BCE and Southeastern Central Europe 5,100-4,000 BCE is – despite greater geographical distance - slightly lower (0.23). The FST between Iran 7,000-five,000 BCE and Southeastern Europe half dozen,200-5,500 BCE is even smaller (0.17). The geographically next groups of Southeastern Europe vi,200-5,500 BCE and 5,500-5,000 BCE and Southeastern Central Europe 5,100-iv,000 BCE reveal a distance as high as 0.16 and 0.ten, respectively.

Population pairwise FSTs between d-loop sequences from eight Neolithic groups of ancient domesticated cattle. Coloured rings environs geographical groups. Grey and white dots inside the circles correspond geographical location of archaeological sites. White dots stand up for the oldest Neolithic samples per grouping, grey dots for Center/Late Neolithic samples. Numbers within dots correspond the number of d-loop sequences per site. Orange: Central/Western Europe five,400-4,400 BCE, blue: Southern France 5,500-4,500 BCE, greenish: Southeastern Cardinal Europe 5,100-iv,000 BCE, yellow: Italy 6,000-5,500 BCE, majestic: Southeastern Europe 6,200-v,500 BCE and five,500-5,000 BCE, cherry-red: Western Anatolia 6,400-five,700 BCE, and grayness: Iran 7,000-v,000 BCE. Numbers on the lines betwixt coloured circles are population pairwise FSTs. Solid lines stand for pregnant FSTs at the 0.05 level, dashed lines stand for significant FSTsouth at the 0.1 level. Grey and white colours of squares on the lines encode which chronological groups per geographi groups are being compared.
A Mantel Test on the basis of Reynolds' FST resulted in a stiff positive correlation (Rxy: 0.75, P-value 0.001) between geographical and genetic distance among the eight groups. Approximately 56% of the variation tin can exist explained past geographical altitude (R2 = 0.56). In that location is a weaker correlation of genetic and geographical distances in modern samples (Rxy: 0.54, P-value 0.002). Here, merely 29% of the variation can exist explained by geographical distance (R2 = 0.29). Complete population pairwise FST matrices can be plant in Additional file 8.
Measurements of molecular diversity (Ĥ, π), Tajima's D and Fu'due south Fs
The estimates of haplotype diversity (Ĥ), the hateful number of pairwise differences (π), Tajima'southward D, and Fu's Fs are given in Tabular array 1.
Haplotype diverseness clearly decreases in a southeast to northwest direction with Islamic republic of iran seven,000-5,000 BCE (0.96) at the loftier end, and Southern France 5,500-iv,500 BCE (0.00) and Fundamental/Western Europe five,400-iv,400 BCE (0.22) at the low cease. The haplotype diversity of the earliest domesticated cattle on the European continent in Southeastern Europe 6,200-5,500 BCE (0.62) is much lower than in Islamic republic of iran, and comparable to Western Anatolia 6,400-5,700 BCE (0.64), but higher than in the geographically close European grouping of Southeastern Central Europe 5,100-four,000 BCE (0.52). Once again, diversity in Central/Western Europe v,400-four,400 BCE is substantially lower (0.22). Following the northern Mediterranean coast, the values also drop sequentially from Western Anatolia six,400-5,700 BCE (0.64) to Italy half-dozen,000-5,500 BCE (0.forty) to Southern France five,500-4,500 BCE (0.00).
Haplotype diversity estimates increase with time in the two regions where samples from ii Neolithic periods are available: from 0.22 to 0.50 in Central/Western Europe and from 0.62 to 0.78 in Southeastern Europe. In Southeastern Europe, haplotype diversity remains the aforementioned in the subsequent Chalcolithic group 5,000-4,000 BCE (0.78), but increases once more during the Bronze Historic period 2,700-ii,200 BCE (0.81). Like patterns are observed when considering the mean numbers of pairwise differences. The Neolithic subgroups also show a tendency of decreasing values with distance from Iran. Regionally, the values increase with fourth dimension; in Central/Western Europe from 0.45 to 0.91 and in Southeastern Europe from ane.26 to 1.79 to 2.11. In the youngest Southeastern European group (2,700-ii,200 BCE) the value drops again (0.54). In Primal/Western Europe, where both diversity indices increase with time, Tajima'south D is also significantly negative (Fu's Fs only in the younger group). This is not the case for Southeastern Europe. Diversity estimates of the 597 modern cattle sequences but bear witness a slight trend towards an east to west slope for both haplotype diversity, and the hateful number of pairwise differences. Tajima'due south D and Fu'south Fs are mostly significantly negative. All diversity estimates and graphical visualisations of chronological and geographical diverseness trends can exist found in the Boosted file 9.
Coalescent simulations
We performed 5 million coalescent simulations under the demographic model described above, and used a tolerance proportion of 0.ane%, meaning that we retained the 5,000 best parameter sets. Effigy 3 shows the joint posterior density of parameters North D and P (marginal to the remaining two), with the articulation fashion found at N D = 81 and P = 0.73. The marginal modal value for N D was 92 (95% credible interval: 29 – 783). Marginal densities for the two migration parameters M E and Thousand L are given in Figure four. While information technology is not possible to infer much from the relatively uninformative posterior for M Eastward (height, mode 0.006 94; 95% CI: 0.00033 – 0.00974), we are able to say that migration between the Near E and Europe (G L ) appears to accept been greatly reduced, essentially to zero, in the menstruation after 5,000 years BCE (lesser, mode 0.00022; 95% CI: 0.00001 – 0.00946). Farther simulations were performed in order to examination the sensitivity of these parameter estimations to our assumed fixed values of Due north NE and Due north East . Increasing or decreasing both North NE and N Eastward by an guild of magnitude produced estimates that did not significantly differ from those given above (see Additional file v for details).
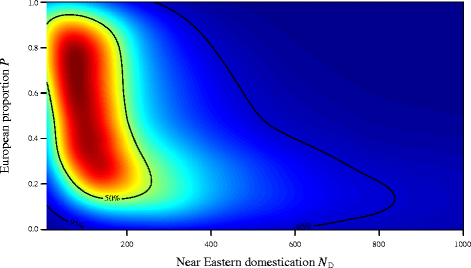
Joint posterior density for the domestication bottleneck (Due north D ) and the proportion moving into Europe (P). The approximate joint posterior probability density of the proportion of the population P allowed to move into Europe at the time of the dissever (vi,400 BCE) and the constructive female person population size at the time of the domestication event (N D ). The 50% and 95% apparent intervals are overlaid equally contours.
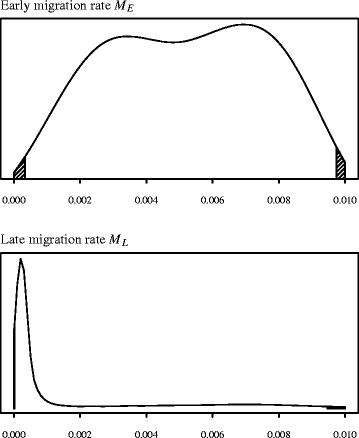
The marginal approximate posterior probability densities of the two migration rate parameters. Chiliad Due east ('early migration') is the rate from the population carve up fourth dimension until five,000 BCE, and Yard Fifty ('belatedly migration') is the rate from 5,000 BCE to nowadays.
Discussion
The domestication process of taurine cattle
Using an ancient (northward = 193) and modern mtDNA dataset (n = 597) of domesticated cattle from the Nigh East, Anatolia and Europe for coalescent simulation and approximate Bayesian computation, we inferred a (joint) posterior manner of 81 female founder individuals at the beginning of the domestication process. This result is consistent with the previous estimate based on xv aboriginal Iranian and 27 mod Most Eastern and Anatolian cattle [23], and demonstrates that this initial finding of a very strong Most Eastern clogging is robust fifty-fifty with a greatly expanded continent-wide data ready, and is non biased by theoretically possible subsequent introgression from aurochs populations exterior the Most East. It can therefore be concluded that domestic cattle indeed have a discrete and rather localised origin, very probable in Southeastern Anatolia and the About Eastward, a view that is consistent with a huge body of archaeozoological testify from the ninethursday millennium BCE [44-46].
Subsequent to the beginning domestication phase, the ancient Deoxyribonucleic acid data, together with archaeological evidence, bespeak to an intermittent expansion scenario. Expanding from Southeastern Anatolia, cattle reached Western Anatolia and the Aegean not earlier 7,000 BCE. From here, they spread simultaneously beyond Southeastern Europe and along the Mediterranean coast into Cardinal, Northern, Southwestern, and Western Europe. In essence, the observed strong correlation between genetic and geographical distances together with decreasing genetic multifariousness roughly in a southeast to northwest and southwest direction is consistent with the idea of serial dilution of diversity by a serial of recurring founder events. The oldest (Neolithic) groups with the greatest geographical distance from each other, namely Iran and Key/Western Europe and Southern France, show the highest FST values (0.47 and 0.4, respectively). Smaller genetic distances are observed between more adjacent areas, such as betwixt Islamic republic of iran and Western Anatolia and betwixt Iran and Southeastern Europe (0.11 and 0.17, respectively). Other statistics are equally consistent with the serial dilution model: Neolithic cattle from Iran yield the highest value for haplotype multifariousness in the whole dataset (0.96). Haplotype diversity consistently decreases along the proposed 2 main Neolithisation routes, with the lowest values in remote areas, i.e. in Neolithic Central/Western Europe and Southern French republic (0.22 and 0.00, respectively), while intermediate values are observed in between.
Culling scenarios of secondary domestications or traceable female gene-menses from wild aurochs in Europe have been discussed several times in the literature [29,47-51]. The arguments are mainly based on deficient findings of the mtDNA haplogroup P, pre-dominating in European aurochs, in the domesticated stock [49,50] on the 1 hand, and the presence of mtDNA lineages in pre-Neolithic Italian aurochs that resemble those of the imported domesticated animals [29,48] on the other, thereby impeding the detection of introgression by mere comparison of haplogroup composition. However, realistic expectations nether such models would likewise include i) a larger inferred founder population due to introgressions of various aurochs lineages and two) pregnant deviations from the serial dilution of genetic diversity model. None of the two has been observed in or can be inferred from the data presented here. Detection of potential introgression of Italian aurochs through fourth dimension deserves further attention, east.g. past expanding the existing dataset to encompass finds from diverse archaeological sites and later on chronological phases. However, the existing dataset from the rest of Europe suggests that introgressions of local genes into the imported domestic cattle populations are rare and geographically restricted exceptions, or coming from male aurochs. Separate contained domestication(s) of European aurochs can about with certainty be excluded.
The strict separation of domestic cattle from their wild European relatives is very different to what can be observed in other animals. For instance, pigs were imported to Europe in a similar mode to cattle, but after a few centuries all their mitochondrial lineages were replaced through admixture with local wild boar [52,53].
The kickoff domesticated cattle in Europe
The summary statistical patterns described here may be partly biased by the fact that the analysed data come from heterochronous and spatially diverse samples [54]. Therefore, nosotros used coalescent simulations to judge the fundamental parameters of taurine cattle population history upon their arrival in Europe in a realistic evolutionary demographic framework.
Our model suggests that a high proportion (73%) of domesticated cattle in Anatolia and the Near East may take migrated into Europe. This indicates that the expansion into Europe was a far less severe bottleneck than assumed, and that much of the variation present in the original Anatolian/Near Eastern population survived in initial European cattle populations. Consistent with this, the Western Anatolian and Southeastern European sample groups constitute a cluster in the MDS plot (Figure one). However, Southern France and Cardinal/Western Europe instead are clearly separated, very likely reflecting genetic diversification along the two primary Neolithisation routes. Information technology is noteworthy that the data from Western Anatolia, Southern Italy, and Southern France come up from very few sites with less than x samples each (eight, 5, and viii, respectively) and therefore have to be evaluated cautiously. Yet, the drastic turn down in haplotype diversity and mean number of pairwise differences from 0.64/0.80 and 0.40/0.79 in Western Anatolia and Italian republic to 0.00/0.00 in Southern France is a skilful fit to a scenario of merely few individuals being transported by gunkhole to the Northwestern Mediterranean coast [3,55]. Low diversity estimates are too congruent with the fact that cattle did non play a major role in the domesticated faunal spectrum of Neolithic economies from Mediterranean Europe (Impressa and Cardial), in contrast to Neolithic Cultures in Cardinal Europe, where domesticated cattle were mostly well represented [56,57].
Tracing the spread of cattle through the European mainland, there are patterns in the data that point to pregnant demographic changes connected to the expansion of the Neolithic civilisation from Southeastern to Central/Western Europe. The genetic distance between Southeastern Europe (6,200-5,500 BCE) and Key/Western Europe (v,400-four,400 BCE) is unexpectedly high (0.27). To put this loftier value into context: the FST values between Iran (seven,000-5,000 BCE), Southeastern Europe (6,200-v,500 BCE) and Italy (half dozen,000-v,500 BCE) are much lower (0.17 and 0.fifteen, respectively) despite larger geographic distances. A proficient indicator for this massive demographic change is that the frequency of the mitochondrial Q-lineage drops from 50 % in Southeastern European (6,200-5,500 BCE) to four% in Central/Western Europe (v,400-iv,400 BCE). Haplotype diversity decreases drastically from 0.62 to 0.22. There are several additional lines of evidence that point to the region betwixt Southeastern Europe and Central Europe as a kind of cadre area where the Neolithic idea was re-consolidated: i) From archeology: The Linearbandkeramik civilization (LBK, engl. Linear Pottery culture) developed here and spread chop-chop over Key Europe starting around 5,600 BCE [58]; ii) From palaeogenetics: A migration of farmers from Southeastern to Central Europe has been inferred using aboriginal mtDNA [xviii]; iii) From gene-culture coevolutionary modelling: Spatially-explicit computer simulations of the spread of an allele associated with lactase persistence in humans (i.e. the power to digest milk saccharide every bit an adult), bespeak to this area as where positive selection started affecting the frequency of this allele in dairying cultures [59]. Nosotros therefore suggest that the observed substantial loss of genetic diversity and the increasing genetic distance in prehistoric cattle are the result of a significant founder event along with the spread of the LBK. Information technology probably coincides with a major wave of man migration and is followed by a period of intensified cattle breeding resulting in a rising importance of dairying. This picture becomes even more comprehensive when we wait at how patterns change afterward the early Neolithic.
Afterward the arrival
Cattle herding becomes more and more than important with the onset of the LBK. A few centuries later, cattle bones plant up to 70% of all domesticated animate being bones in faunal assemblages in Key Europe, a value that stays roughly the same for nigh of the subsequent millennia with some regional fluctuations [56,57]. Accordingly, significantly negative Tajima'due south D and Fu'south Fs values in Neolithic Cardinal/Western Europe and in the majority of the modern sample groups bespeak to extended periods of population growth (see Table one and Boosted file 9).
Interestingly, there is no indication for population growth in Southeastern Europe. The observed diachronic increment in haplotype variety in the Southeastern European sample groups appears in tandem with new, previously absent mitochondrial haplotypes (too meet Additional file 7). It is of notation that ii of these new haplotypes (T_H6 and T2_H7) are present here and in the Iranian Neolithic sample simply not elsewhere.
Co-ordinate to our demographic modelling, migration between Anatolia/the Near E and Europe was greatly reduced, substantially to zero, in the catamenia after 5,000 BCE. We should expect that accurately estimating the level of early migration between 6,400 and 5,000 BCE to be hard, equally it is somewhat confounded by the proportion of cattle P moving to Europe at the time of the dissever (indeed the ii parameters are very slightly negatively correlated; degree r = -0.07, p = 0.0002). However, it is articulate that there is support at least for some level of migration during this early menstruum every bit the estimated modal migration rate is clearly greater than 0.
We therefore suggest that a probable underlying scenario for our observations is one of continuous gene-flow into Europe following the initial colonization at effectually 6,400 BCE. This scenario also fits in with archaeological prove for accelerated due west acculturation occurring in the first half of the half dozenth millennium BCE [6,60]. This early on phase was followed by almost full isolation between the European and Anatolian/Near Eastern cattle populations afterward 5,000 BCE.
It is of notation that this blueprint has inverse again in later periods. The pattern of decreasing diversity in the management of the Neolithic expansion and the correlation of genetic and geographical distances is considerably weaker in modern-24-hour interval cattle breeds than in the Neolithic. Information technology is non clear still to which extent human migrations from the East as postulated for the Bronze Age [61] influenced the already existing cattle stock in Europe. Still, the fading geographical patterns are likely mirroring more contempo demographic changes and founder events, such equally global trade, exceptional selection pressure on particular high performance breeds and replacement of traditional breeds [62]. Thus, the present study explicitly underlines that ancient demographic and evolutionary processes in selectively bred animals tin can only be uncovered by using ancient Deoxyribonucleic acid data.
Conclusions
Overall, palaeogenetic together with archaeological and archaeozoological data strongly support the following scenario: taurine cattle were domesticated in a region between Southeastern Anatolia and the Zagros Mountains, Syrian arab republic and the Lebanon. The domestication process started in the mid-9thursday millennium BCE, with a small effective number of wild female aurochs (estimated modal value of 81). Afterward vii,000 BCE, domestic cattle populations were transported from the Central Anatolian plateau to Western Anatolia and the Aegean. Much of the original Anatolian and Well-nigh Eastern variation (approximately 73%) survived in the first Neolithic cattle that were introduced to Europe around 6,400 BCE. Despite some evidence for subsequent gene-menstruation with Anatolia and the Well-nigh East betwixt 6,400 and five,000 BCE, most of the initial genetic diversity was lost as cattle spread through Europe along with the Neolithic transition: Via the Mediterranean trajectory, migrating farmers reached i.e. Southern Italy, Northern Africa, the Tyrrhenian Islands, Southern France and the Iberian Peninsula by gunkhole. The low genetic diversity observed in the few genetic data bachelor from these regions points to a significantly depression effective population size of cattle arriving in the Western Mediterranean. Along the 2nd trajectory beyond the European mainland and without major signs of introgression from wild aurochs, cattle finally reached Key, Western (after 5,500 BCE) and Northern Europe (after 4,100 BCE). Also here, much of the genetic diversity was lost during the move, particularly when cattle were brought to Central Europe by LBK farmers.
Cistron-flow between Europe and Anatolia and the Near Due east appears to have been reduced, essentially to 0, after around 5,000 BCE. In modern breeds however, the genetic furnishings of the inferred migratory patterns and geographical diversification become far less pronounced, probably due to selective breeding and trade of loftier performance cows in very recent times.
In summary, the genetic prehistory of domestic cattle seems to consist of a small, localised domestication procedure, followed past a relatively straightforward series of spasmodic expansion episodes resulting in a serial dilution of genetic diversity from the Near E to Western and Northern Europe. Future genomic multi-locus studies of ancient Deoxyribonucleic acid from prehistoric periods will hopefully add greater detail to this picture, peculiarly by incorporating the potentially divergent census of male cattle.
Reference
-
Özdoğan M. The expansion of the neolithic fashion of life: What nosotros know and what we exercise non know. In: How did farming reach Europe? Edited by Lichter C, vol. 2. Istanbul: Byzas; 2005. p. thirteen–27.
-
Özdoğan Grand. Archaeological Evidence on the West Expansion of Farming Communities from Eastern Anatolia to the Aegean and the Balkans. Curr Anthropol. 2011;52(S4):S415–xxx.
-
Vigne J-D. Zooarchaeological aspects of the Neolithic diet transition in the Well-nigh East and Europe, and their putative relationships with the Neolithic Demographic Transition. In: Bocquet-Appel J-P OB-Y, editor. The Neolithic Demographic Transition and its Consequences. New York: Springer Verlag; 2008. p. 179–205.
-
Conolly J, Colledge S, Dobney Chiliad, Vigne J-D, Peters J, Stopp B, et al. Meta-analysis of zooarchaeological information from SW Asia and SE Europe provides insight into the origins and spread of creature husbandry. J Archaeol Sci. 2011;38(3):538–45.
-
Özdoğan M. An alternative approach in tracing changes in demographic composition. The westward expansion of the neolithic way of life. In: Bocquet-Appel J-P, Bar-Yosef O, editors. The neolithic demographic transition and its consequences. 2008th ed. Berlin: Springer; 2008. p. 139–78.
-
Düring BS. The prehistory of Asia Minor : from circuitous hunter-gatherers to early on urban societies. Cambridge: Cambridge University Press; 2011.
-
Çilingiroğlu A, Cevik O, Çilingiroğlu C. Ulucak Höyük: Towards understanding the early farming communities of Middle West Anatolia: Contribution of Ulucak. In: Özdoğan M, Başgelen Due north, Kuniholm P, editors. The Neolithic in Turkey, Western Turkey. Istanbul: Archæology & Art Publications; 2012. p. 139–75.
-
Reingruber A. Die deutschen Ausgrabungen auf der Agrissa-Magula in Thessalien Ii. Dice Agrissa Magula. In: Beiträge zur ur- und frühgeschichtlichen Archäologie des Mittelmeer-Kulturraums, Hauptmann H, editors. Das frühe und das beginnende mittlere Neolithikum im Lichte transägäischer Beziehungen. Bonn: Dr. Rudolf Habelt GmbH; 2008.
-
Lüning J. Steinzeitliche Bauern in Federal republic of germany – die Landwirtschaft im Neolithikum. Bonn: Dr. Rudolf Habelt GmbH; 2000.
-
Guilaine J. De la vague à la tombe, La conquête néolithique de la Méditerranée (8000-2000 avant J.-C). In. Paris: Le Seuil; 2003.
-
Tresset A, Vigne J-D. Last hunter-gatherers and get-go farmers of Europe. C R Biol. 2011;334(3):182–9.
-
Ammerman AJ, Cavalli-Sforza LL. The Neolithic transition and the genetics of populations in Europe. Princeton, Guildford: Princeton University Press; 1984.
-
Zvelebil Grand, Zvelebil KV. Agricultural transition and Indo-European dispersals. Artifact. 1988;62(236):574–83.
-
Ammerman AJ. On the Neolithic Transition in Europe - a Comment. Antiquity. 1989;63(238):162–5.
-
Zvelebil M. On the Transition to Farming in Europe, or What Was Spreading with the Neolithic - a Respond. Antiquity. 1989;63(239):379–83.
-
Whittle AWR. Europe in the Neolithic : the creation of new worlds. Cambridge: Cambridge University Press; 1996.
-
Pinhasi R, Thomas MG, Hofreiter One thousand, Currat M, Burger J. The genetic history of Europeans. Trends Genet. 2012;28(10):496–505.
-
Bramanti B, Thomas MG, Haak W, Unterlaender Thousand, Jores P, Tambets Thousand, et al. Genetic discontinuity between local hunter-gatherers and central Europe's kickoff farmers. Science. 2009;326(5949):137–40.
-
Skoglund P, Malmstrom H, Raghavan M, Stora J, Hall P, Willerslev E, et al. Origins and genetic legacy of Neolithic farmers and hunter-gatherers in Europe. Scientific discipline. 2012;336(6080):466–9.
-
Lazaridis I, Patterson Due north, Mittnik A, Renaud G, Mallick Due south, Kirsanow Chiliad, et al. Ancient human genomes suggest iii ancestral populations for present-day Europeans. Nature. 2014;513(7518):409–13.
-
Tresset A, Bollongino R, Edwards CJ, Hughes Due south, Vigne J-D. Early improvidence of domestic bovids in Europe: An indicator for human contact, exchanges and migrations? In: Hombert JM, Errico F, editors. Becoming eloquent, advances in the emergence of linguistic communication, human being cognition, and modern cultures. Amsterdam: John Benjamins Publ. Comp; 2009. p. 69–90.
-
Larson G, Burger J. A population genetics view of creature domestication. Trends Genet. 2013;29(iv):197–205.
-
Bollongino R, Burger J, Powell A, Mashkour M, Vigne J-D, Thomas MG. Modernistic Taurine Cattle descended from small-scale number of Virtually-Eastern founders, Molecular Biology and Evolution. 2012. doi:10.1093/molbev/mss1092.
-
Bollongino R, Edwards CJ, Alt KW, Burger J, Bradley DG. Early history of European domestic cattle equally revealed by aboriginal DNA. Biol Lett. 2006;2(i):155–9.
-
Anderung C, Bouwman A, Persson P, Carretero JM, Ortega AI, Elburg R, et al. Prehistoric contacts over the Straits of Gibraltar indicated by genetic analysis of Iberian Statuary Historic period cattle. Proc Natl Acad Sci U S A. 2005;102(24):8431–5.
-
Edwards CJ, Bollongino R, Scheu A, Chamberlain A, Tresset A, Vigne J-D, et al. Mitochondrial Deoxyribonucleic acid analysis shows a Nearly Eastern Neolithic origin for domestic cattle and no indication of domestication of European aurochs. Proc R Soc B Biol Sci. 2007;274(1616):1377–85.
-
Scheu A, Hartz Due south, Schmoelcke U, Tresset A, Burger J, Bollongino R. Aboriginal Deoxyribonucleic acid provides no evidence for independent domestication of cattle in Mesolithic Rosenhof. Northern Germany Journal of Archaeological Scientific discipline. 2008;35(v):1257–64.
-
Bollongino R, Elsner J, Vigne J-D, Burger J. Y-SNPs practice not indicate hybridisation betwixt European aurochs and domestic cattle. PLoS One. 2008;3(10), e3418.
-
Beja-Pereira A, Caramelli D, Lalueza-Fox C, Vernesi C, Ferrand N, Casoli A, et al. The origin of European cattle: evidence from modern and aboriginal Dna. Proc Natl Acad Sci U S A. 2006;103(21):8113–8.
-
Troy CS, MacHugh DE, Bailey JF, Magee DA, Loftus RT, Cunningham P, et al. Genetic show for Nigh-Eastern origins of European cattle. Nature. 2001;410(6832):1088–91.
-
Excoffier L, Lischer HE. Arlequin suite ver 3.5: a new serial of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Resour. 2010;10(iii):564–7.
-
R Developement Core Team. R: A Language and Surround for Statistical Calculating. R Foundation for Statistical Calculating, Vienna, Austria, R Foundation for Statistical Computing. 2012. ISBN: 3-900051-07-0, URL http://www.R-project.org/.
-
Venables WN, Ripley BD. Modern Practical Statistics with S. quaternary ed. New York: Springer Verlag; 2002.
-
Lemon J. Plotrix: a parcel in the red light district of R. R-News. 2006;6(4):8–12.
-
Soetaert Thousand: Shape: Functions for plotting graphical shapes, colors. R package version 1.3.4. http://CRAN.R-project.org/package=shape. 2011.
-
Mantel Northward. The detection of disease clustering and a generalized regression approach. Cancer Res. 1967;27:209–twenty.
-
Peakall R, Smouse PE. GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and inquiry Molecular Environmental Notes. 2006;6(1):288–95.
-
Anderson CN, Ramakrishnan U, Chan YL, Hadly EA. Serial SimCoal: a population genetics model for data from multiple populations and points in time. Bioinformatics. 2005;21(8):1733–iv.
-
MacEachern S, Hayes B, McEwan J, Goddard K. An examination of positive selection and changing effective population size in Angus and Holstein cattle populations (Bos taurus) using a loftier density SNP genotyping platform and the contribution of ancient polymorphism to genomic variety in Domestic cattle. BMC Genomics. 2009;10:181.
-
Beaumont MA, Zhang Westward, Balding DJ. Gauge Bayesian computation in population genetics. Genetics. 2002;162(iv):2025–35.
-
Achilli A, Bonfiglio Southward, Olivieri A, Malusa A, Pala One thousand, Hooshiar Kashani B, et al. The multifaceted origin of taurine cattle reflected by the mitochondrial genome. PLoS One. 2009;4(half-dozen), e5753.
-
Gravlund P, Aaris-Sorensen K, Hofreiter M, Meyer K, Bollback JP, Noe-Nygaard North. Ancient Deoxyribonucleic acid extracted from Danish aurochs (Bos primigenius): genetic diversity and preservation. Ann Anat. 2012;194(1):103–11.
-
Lenstra J, Ajmone-Marsan P, Beja-Pereira A, Bollongino R, Bradley D, Colli L, et al. Meta-Analysis of Mitochondrial DNA Reveals Several Population Bottlenecks during Worldwide Migrations of Cattle. Diversity. 2014;6(1):178–87.
-
Peters J, von den Driesch A, Helmer D. The upper Euphrates-Tigris basin: Cradle of agro-pastoralism? In: Vigne J-D, Helmer D, editors. The outset steps of animal domestication New archaeological approaches Proceedings of the ninth ICAZ Conference, Durham 2002. Oxford: Oxbow Books; 2005. p. 96–124.
-
Helmer D, Gourichon L, Monchot H, Peters J, Segui MS. Identifying early domestic cattle from Pre-Pottery Neolithic sites on the Middle Euphrates using sexual dimorphism. In: Vigne J-D, Peters J, Helmer D, editors. The beginning steps of animal domestication New archaeological approaches Proceedings of the 9th ICAZ Conference, Durham 2002. Oxford: Oxbow Books; 2005. p. 86–95.
-
Hongo H, Pearson J, Öksük B, Ilgezdi Thou. The procedure of ungulate domestication at Çayönü. Southeastern Turkey: A multidisciplinary approach focusing on Bos sp and Cervus elaphus Anthropozoologica. 2009;44:63–73.
-
Achilli A, Olivieri A, Pellecchia M, Uboldi C, Colli L, Al-Zahery N, et al. Mitochondrial genomes of extinct aurochs survive in domestic cattle. Curr Biol. 2008;18(4):R157–8.
-
Mona S, Catalano G, Lari Thousand, Larson Thousand, Boscato P, Casoli A, et al. Population dynamic of the extinct European aurochs: genetic show of a northward-s differentiation design and no evidence of post-glacial expansion. BMC Evol Biol. 2010;x:83.
-
Schibler J, Elsner J, Schlumbaum A. Incorporation of aurochs into a cattle herd in Neolithic Europe: unmarried consequence or breeding? Sci Rep. 2014;4:5798.
-
Stock F, Edwards CJ, Bollongino R, Finlay EK, Burger J, Bradley DG. Cytochrome b sequences of ancient cattle and wild ox support phylogenetic complexity in the ancient and modern bovine populations. Anim Genet. 2009;twoscore(five):694–700.
-
Bonfiglio Due south, Achilli A, Olivieri A, Negrini R, Colli L, Liotta L, et al. The enigmatic origin of bovine mtDNA haplogroup R: sporadic interbreeding or an independent event of Bos primigenius domestication in Italian republic? PLoS 1. 2010;5(12), e15760.
-
Ottoni C, Flink LG, Evin A, Geörg C, De Cupere B, Van Neer Westward, et al. Pig domestication and human-mediated dispersal in western Eurasia revealed through ancient DNA and geometric morphometrics. Mol Biol Evol. 2013;thirty(4):824–32.
-
Geörg C. Paläopopulationsgenetik von Schwein und Schaf in Südosteuropa und Transkaukasien. ForschungsCluster1. vol. 9. Verlag Marie Leidorf GmbH: Rahden/Westf; 2013.
-
Depaulis F, Orlando Fifty, Hanni C. Using Classical Population Genetics Tools with Heterochroneous Information. Time Matters! PLoS Ane. 2009;four(five):5541.
-
Guilaine J, Manen C. Vigne J-D. Pont de Roque-Haute (Portiragnes, Hérault). Nouveaux regards sur la néolithisation de la France méditerranéenne. Archives d'Ecologie Préhistorique: Toulouse; 2007.
-
Benecke Northward. Der Mensch und seine Haustiere. Die Geschichte einer jahrtausendealten Beziehung. Stuttgart: Theiss; 1994.
-
Tresset A, Vigne J-D. La chasse, principal élément structurant la diversité des faunes archéologiques du Néolithique ancien, en Europe tempérée comme en Méditerranéenne: tentative d'interprétation fonctionnelle. In: Arbogast RM, Jeunesse C, Schibler J, editors. Rôle et statut de la chasse dans le Néolithique ancien danubien (5500-4900 av J-C) Actes Premières rencontres danubiennes de Strasbourg, 20-21 nov 96. Rahden/Westf: Marie Leidorf; 2001. p. 129–51.
-
Pavúk J. Typologische Geschichte der Linearbandkeramik. In: Lüning J, Frirdich C, Zimmermann A, editors. Dice Bandkeramik im 21 Jahrhundert: Symposium in der Abtei Brauweiler bei Köln 2002. Rahden/Westf: Verlag Marie Leidorf GmbH; 2005. p. 17–39.
-
Itan Y, Powell A, Beaumont MA, Burger J, Thomas MG. The origins of lactase persistence in Europe. PLoS Comput Biol. 2009;5(8), e1000491.
-
Çilingiroğlu C. The appareance of impressed pottery in the Neolithic Aegean and its implications for maritime networks in the Eastern Mediterranean. Tüba-Ar. 2010;13:9–22.
-
Haak W, Lazaridis I, Patterson N, Rohland N, Mallick S, Llamas B, et al. Massive migration from the steppe was a source for Indo-European languages in Europe, Nature. 2015.
-
Taberlet P, Coissac E, Pansu J, Pompanon F. Conservation genetics of cattle, sheep, and goats. C R Biol. 2011;334(three):247–54.
Acknowledgements
This work was funded by German Archaeological Found, Johannes Gutenberg-University Mainz, and CNRS (Centre national de la recherche scientifique).
The authors would like to thank Adrian Bӑlӑşescu, Cornelia Becker, Daniel Bradley, Altan Çilingiroğlu, Çiler Çilingiroğlu, Giuliano Cremonesi, Keith Dobney, Ceiridwen Edwards, Ralf Gleser, Angela Graefen, Jean Guilaine, Svend Hansen, Daniel Helmer, Robert Hofmann, Raiko Krauß, Marion Lichardus-Itten, Claire Manen, Ingo Motzenbäcker, Mehmet Özdoğan, Jean Roudil, Silviane Scharl, Nils Müller-Scheeßel, Wolfram Schier, Mona Schreiber, Elisabeth Stephan, and Bernhard Weninger for providing bone material and for helpful discussions.
Author information
Affiliations
Respective author
Additional information
Competing interests
The authors declare that they take no competing interests.
Authors' contributions
JB, NB, and JDV designed the research, AS and RB performed the experiments, AS, RB, AP, JDV, AT, CC, NB, and JB analyzed and interpreted data, and AS, AP, and JB wrote the newspaper. All authors read and approved the final version of the paper.
Additional files
Additional file one:
Sample list. Supplemental data on the location of the archaeological sites, dating, sample providers, sequencing results, and GenBank accession numbers of all new sequences.
Additional file ii:
GenBank accession numbers. GenBank accession numbers and references of all previously published ancient and modernistic mtDNA sequences used in this study. Modern sequences are grouped geographically by their country of origin.
Additional file 3:
Ancient Deoxyribonucleic acid methods. Supplemental methodological detail on DNA amplification and Deoxyribonucleic acid sequencing (such as primer sequences and locations, and detailed protocols), haplogroup determination, and establishment of consensus sequences.
Additional file 4:
Chronological and geographical sample groups. Individual assignment of all aboriginal sequences used in this written report to chronological, cultural and geographical groups, and individual haplogroup and haplotype assignments and polymorphic positions. The relative frequency of halplogroup Q per group is provided with a 95% confidence interval (CI). Shared haplotypes (H) are numbered consecutively. Haplogroup T stands for T, T5 or T1'2'3. Haplogroup consignment co-ordinate to [41]. Polymorphic positions are given according to the reference sequence GenBank V00654, whereby dots represent bases that match the reference sequence, and asterisks unavailable sequence information.
Additional file 5:
Coalescent simulations and ABC. Methodological detail on the coalescent-based demographic modelling of the domestication and and so spread of cattle into Europe.
Additional file six:
Validation of aboriginal DNA data. Supplemental information on ancient DNA validity criteria (such as blank controls and contamination charge per unit estimation), and explicit discussion of not fully replicated sequences.
Additional file 7:
Shared haplotypes. Shared haplotypes (H) are named and coloured according to their haplogroup and numbered consecutively. Shades of cherry: T3; White/light greyness: Q; Shades of blueish: T, T5 or T1'2'3; Shades of green: T2 (haplogroup definition according to [41]). The x-axis gives the number of sequences. a) Haplotype distribution across all 193 aboriginal mtDNA sequences; b) Haplotype distribution beyond 13 spatiotemporal groups defined by region of origin and age in BCE to the left of each bar.
Additional file eight:
F ST values. a) Ancient pairwise FSTsouthward. b) Ancient pairwise FST P values. c) Ancient Reynolds' FSTdue south. d) Modern pairwise FSTs. eastward) Modern pairwise FST P values. For a)-c): First row/column:IR/S: Islamic republic of iran/Syrian arab republic; Information technology: Italy; SECE: Southeastern Central Europe; SEE: Southeastern Europe; SF: Southern France; SP: Spain; WA: Western Anatolia; CWE: Central/Western Europe.Numbers betoken the age of the samples in BCE; numbers in brackets betoken sample size.Significant values at the 0.05 level are highlighted in bold. For d)-eastward): Beginning row/column: Expanse/state of origin. Numbers in brackets signal sample size. Significant values at the 0.05 level are highlighted in bold.
Additional file ix:
Graphical representation of Table 1 and summary statistics from modern mtDNA sequences. a) Boxplots of aboriginal haplotype variety. b) Boxplot of ancient mean numbers of pairwise differences. Estimates are sorted by the magnitude of the oldest available chronological group per geographical group. Markers are colored by chronology as follows: white: (primeval) Neolithic, grey: Heart/Late Neolithic, dashed: Chalcolithic, black: post-Neolithic. Ten -axis: IR/S: Islamic republic of iran/Syria, IT: Italy, SECE: Southeastern Central Europe, Meet: Southeastern Europe, SF: Southern France, WA: Western Anatolia, and CWE: Central/Western Europe. Numbers bespeak the age of the samples in BCE; numbers in brackets bespeak sample size. c) Summary statistics of d-loop sequences from 11 geographical groups of modern domesticated cattle. First cavalcade: area/state of origin. Ĥ: Haplotype diversity, π: mean number of pairwise differences. Pregnant Tajima's D and Fu'due south Fs value at the 0.05 level are highlighted in bold. d) Boxplot of modern haplotype diversity. eastward) Boxplot of modern mean numbers of pairwise differences. The boxplots are sorted by magnitude. X-axis: country/area of origin; numbers in brackets betoken sample size.
Rights and permissions
This is an Open Access article distributed under the terms of the Creative Eatables Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/nada/1.0/) applies to the information made bachelor in this commodity, unless otherwise stated.
Reprints and Permissions
Well-nigh this article
Cite this commodity
Scheu, A., Powell, A., Bollongino, R. et al. The genetic prehistory of domesticated cattle from their origin to the spread across Europe. BMC Genet sixteen, 54 (2015). https://doi.org/x.1186/s12863-015-0203-2
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/x.1186/s12863-015-0203-ii
Keywords
- Haplotype Diversity
- Estimate Bayesian Ciphering
- Lactase Persistence
- Domesticate Cattle
- Coalescent Simulation
Source: https://bmcgenomdata.biomedcentral.com/articles/10.1186/s12863-015-0203-2
Posted by: holtmanlepaso.blogspot.com

0 Response to "Where Did The Europeans Get Their Domesticated Animals From"
Post a Comment